WHY IS THE OCEAN SALTLY?

How salty is the ocean?
Some scientists estimate that the oceans contain as much as 50 quadrillion tons (50 million billion tons) of dissolved solids.
If the salt in the sea could be removed and spread evenly over the Earth’s land surface it would form a layer more than 500 feet thick, about the height of a 40 story office building. The saltiness of the ocean is more understandable when compared with the salt content of a fresh-water lake. For example, when 1 cubic foot of sea water evaporates it yields about 2.2 pounds of salt, but 1 cubic foot of fresh water from Lake Michigan contains only one one-hundredth (0.01) of a pound of salt, or about one sixth of an ounce. Thus, sea water is 220 times saltier than the fresh lake water.
The origin of the sea
Ocean and sea are used interchangeably. Today’s seas are the North and South Pacific, North and South Atlantic, Indian and Arctic Oceans and the Antarctic waters of seas. There are several theories about the origin of the seas, but no single theory explains all aspects of this puzzle. Many earth scientists agree with the hypothesis that both the atmosphere and the oceans have accumulated gradually through geologic time from some process of "degassing” of the Earth’s interior. According to this theory, the ocean had its origin from the prolonged escape of water vapour and other gases from the molten igneous rocks of the Earth to the clouds surrounding the cooling Earth. After the Earth’s surface had cooled to a temperature below the boiling point of water, rain began to fall and continued to fall for centuries. As the water drained into the great hollows in the Earth’s surface, the primeval ocean came into existence. The forces of gravity prevented the water from leaving the planet.
Sources of the salts
Sea water has been defined as a weak solution of almost everything. Ocean water is indeed a complex solution of mineral salts and of decayed biologic matter that results from the teeming life in the seas. Most of the ocean’s salts were derived from gradual processes such the breaking up of the cooled igneous rocks of the Earth’s crust by weathering and erosion, the wearing down of mountains, and the dissolving action of rains and streams which transported their mineral washings to the sea. Some of the ocean’s salts have been dissolved from rocks and sediments below its floor. Other sources of salts include the solid and gaseous materials that escaped from the Earth’s crust through volcanic vents or that originated in the atmosphere.
In the beginning
Primeval seas must have been only slightly salty. But ever since the first rains descended upon the young Earth hundreds of millions of years ago and ran over the land breaking up rocks and transporting their minerals to the seas, the ocean has become saltier. It is estimated that the rivers and streams flowing from the United States alone discharge 225 million tons of dissolved solids and 513 million tons of suspended sediment annually to the sea. Throughout the world, rivers carry an estimated 4 billion tons of dissolved salts to the ocean annually. About the same tonnage of salt from the ocean water probably is deposited as sediment on the ocean bottom, and thus, yearly gains may offset yearly losses. In other words, the oceans today probably have a balanced salt input and outgo.
Past accumulations of dissolved and suspended solids in the sea do not explain completely why the ocean is salty. Salts become concentrated in the sea because the Sun’s heat distils or vaporizes almost pure water from the surface of the sea and leaves the salts behind. This process is part of the continual exchange of water between the Earth and the atmosphere that is called the hydrologic cycle. Water vapour rises from the ocean surface and is carried landward by the winds. When the vapour collides with a colder mass of air, it condenses and falls to Earth as rain. The rain runs off into streams which in turn transport water to the ocean. Evaporation from both the land and the ocean again causes water to return to the atmosphere as vapour and the cycle starts anew. The ocean, then, is not fresh like river water because of the huge accumulation of salts by evaporation and the contribution of raw salts from the land. In fact, since the first rainfall, the seas have become saltier.
Sea water is not simple
Scientists have studied the ocean’s water for more than a century, but they still do not have a complete understanding of its chemical composition. This is partly due to the lack of precise methods and procedures for measuring the constituents in sea water. Some of the problems confronting scientists stem from the enormous size of the oceans, which cover about 70 percent of the Earth’s surface, and the complex chemical system inherent in a marine environment in which constituents of sea water have intermingled over vast periods of time. At least 72 chemical elements have been identified in sea water, most in extremely small amounts.
Salinity and its variability
Oceanographers report salinity (total salt content) and the concentrations of individual chemical constituents in sea water—chloride, sodium, or magnesium for example in parts per thousand, for which the symbol ο/οο is used. Ex: 35 ο/οο means 35 pounds of salt per 1,000 pounds of sea water. The salinity of ocean water varies. It is affected by such factors as melting of ice, inflow of river water, evaporation, rain, snowfall, wind, wave motion, and ocean currents that cause horizontal and vertical mixing of the saltwater.
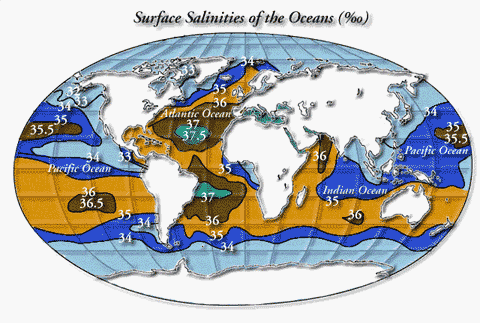
The saltiest water
The saltiest water (40 ο/οο) occurs in the Red Sea and the Persian gulf, where rates of evaporation are very high. Of the major oceans, the North Atlantic is the saltiest; its salinity averages about 37.9 ο/οο. Within the North Atlantic, the saltiest part is the Sargasso Sea. The Sargasso Sea is set apart from the open ocean by floating brown seaweed "sargassum” from which the sea gets its name. The saltiness of this sea is due in part to the high water temperature (up to 83º F (28.3)) causing a high rate of evaporation and in part to its remoteness from land; because it is so far from land, it receives no fresh-water inflow.
Low salinities occur in polar seas where the salt water is diluted by melting ice and continued precipitation. Partly landlocked seas or coastal inlets that receive substantial runoff from precipitation falling on the land also may have low salinities.
- The Baltic Sea ranges in salinity from about 5 to 15 ο/οο
- The Black Sea is less than 20 ο/οο
- Water of the Puget Sound in the Tacoma Wash., area ranges in salt content from 21 to about 27 ο/οο (This area is drained by a number of fresh-water streams which discharge an average of about 4.1 billion gallons of water per day into Puget Sound).
The water off the coast of Miami Beach, Florida has a high salt content because it is undiluted sea water. Off the coast of Astoria, however, the sea water is less saline because it is mixed with the fresh water of the mighty Columbia.
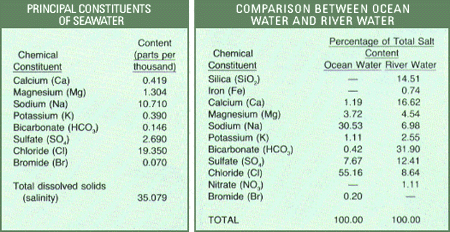
The salt content of the open oceans, free from land influences, is rarely less than 33 ο/οο and seldom more than 38 ο/οο. Throughout the world, the salinity sea water averages about 35 ο/οο. This average salinity was obtained by William dittmar in 1884 from chemical analyses of 77 sea water samples collected from many parts of the world during the scientific expedition of the British corvette, H.M.S. Challenger. The Challenger expedition, organized by the British Government at the suggestion of the Royal Society, set out to study the biology of the sea, examine the chemical and physical properties of the water, sample deposits on the ocean floor, and measure water temperatures. The voyage began in 1872 and ended almost 4 years later after covering 68,890 nautical miles. This expedition remains today the longest continuous scientific investigation of the ocean basins. Dittmar’s 77 samples are still the only worldwide set of samples of sea water for which complete data (each principal constituent) on chemical composition are available. More recent data, reflecting improvements in analytical and sampling techniques, show slight deviations from Dittmar’s results, but these changes do not affect the overall usefulness of his work. The average composition of the 77 samples is as shown on the following table.
The salinity of water in the open sea is not fixed at 35 ο/οο even in areas distant from land; that figure is only an average. On a worldwide basis, a maximum salinity of 36 ο/οο occurs at about latitudes 20º N. and 20º S. The average salinity of sea water, 35 ο/οο, occurs at the Equator. A minimum salinity of 31 ο/οο corresponds approximately with latitude 60º N., whereas lowest salinities of 33 ο/οο in the Southern Hemisphere occur at latitude 60º S. At the Equator, where salinity is 35 ο/οο, the dilution of sea water by rain is offset by the loss of water by evaporation. But in the latitudes bordering the Equator the opposite condition prevails—evaporation exceeds rainfall because high temperatures plus increased winds accelerate evaporation losses.
Facts about sea water and river water
- Sodium and chloride (the components of common table salt) constitute a little more than 85 percent of the dissolved solids in ocean water and give to the water its characteristic salty taste, but they represent less than 16 percent of the salt content of river water.
- Rivers carry to the sea more calcium than chloride, but the oceans nevertheless contain about 46 times more chloride than calcium.
- Silica is a significant constituent of river water but not of sea water.
- Calcium and bicarbonate account for nearly 50 percent of the dissolved solids in river water yet constitute less than 2 percent of the dissolved solids in ocean water. These variations seem contrary to what one would expect.
Part of the explanation is the role played by marine life—animals and plants—in ocean water’s composition. Sea water is not simply a solution of salts and dissolved gases unaffected by living organisms in the sea.
- Mollusks (oysters, clams, and mussels) extract calcium from the sea to build their shells and skeletons.
- Foraminifers (very small one-celled sea animals) and crustaceans (such as crab, shrimp, lobster, and barnacles) likewise take out large amounts of calcium salts to build their bodies.
- Coral reefs, common in warm tropical seas, consist mostly of limestone (calcium carbonate) formed over millions of years from the skeletons of billions of small corals and other sea animals.
- Plankton (tiny floating animal and plant life) also exerts control on the composition of sea water.
- Diatoms, members of the plankton community, require silica to form their shells and they draw heavily on the ocean’s silica for this purpose.
Some marine organisms
concentrate or secrete chemical elements that are present in such minute
amounts in sea water as to be almost undetectable.
- Lobsters concentrate copper and cobalt.
- Snails secrete lead.
- The sea cucumber extracts vanadium.
- Sponges and certain seaweeds remove iodine from the sea.
Sea life has a strong influence on the composition of sea water. However, some elements in sea water are not affected to any apparent extent by plant or animal life. For Example, no known biological process removes the element sodium from the sea.
In addition to biological influences, the factors of solubility and physical-chemical reaction rates also help to explain the composition of sea water. The solubility of a constituent may limit its concentration in sea water. Excess calcium (more calcium that the water can hold) may be precipitated out of the water and deposited on the sea floor as calcium carbonate. Presumably as a result of physical-chemical reactions not well understood, the metal manganese occurs as nodules in many places on the ocean floor. Similarly, phosphorite (phosphate rock) is found in large amounts on the sea bottom off southern California and in lesser amounts in several other places.
Near-constant ratios of major constituents
Although the composition of sea water differs from that of river water, the proportions of the major constituents of sea water are almost constant throughout the world. Dittmar’s 77 samples showed no significant global differences in relative composition, and his average concentrations are used today to represent the ratios of major constituents in sea water. The analyses, which Dittmar made over a period of 9 years, further showed that chloride, sodium, magnesium, sulphate, calcium, and potassium make up 99 percent of the dissolved solids in sea water. Dittmar’s findings may be expressed in another way: although the salinity or total salt content may vary from place to place, the ratio of any one major constituent of sea water (chloride as an example) to the total content is nearly constant. However, the ratios of the less abundant elements (aluminum, copper, tin, and bismuth, for example) to total salt content are not constant nor are those of dissolved gases such as oxygen, carbon dioxide, and nitrogen. But establishment of the near constancy of the ratios of major constituents of sea water is important because it enables scientists to measure one principal element and then, by projection of ratios and correction for temperature and pressure, to calculate the other components in the water, thereby determining its salinity.
Conclusion
The ocean is salty because of the gradual concentration of dissolved chemicals eroded from the Earth’s crust and washed into the sea. Solid and gaseous ejections from volcanoes, suspended particles swept to the ocean from the land by onshore winds, and materials dissolved from sediments deposited on the ocean floor have also contributed. Salinity is increased by evaporation or by freezing of sea ice and it is decreased as a result of rainfall, runoff, or the melting of ice. The average salinity of sea water is 35 ο/οο, but concentrations as high as 40 ο/οο are observed in the Red Sea and the Persian Gulf. Sea water not only is much saltier than river water but it also differs in the proportion of the various salts. Sodium and chloride constitute 85 percent of the dissolved solids in sea water and account for the characteristic salty taste. In open water the chemical composition of sea water is nearly constant. Because of the stable ratios of the principal constituents to total salt content, the determination of one major constituent can be used to calculate sea water salinity. For minor constituents and dissolved gases the composition is variable and therefore ratios cannot be used to calculate salt. Circulation and mixing, density and ocean currents, wind action, water temperature, solubility, and biochemical reactions are some of the factors that explain why the composition of water in the open sea is almost constant from place to place.


