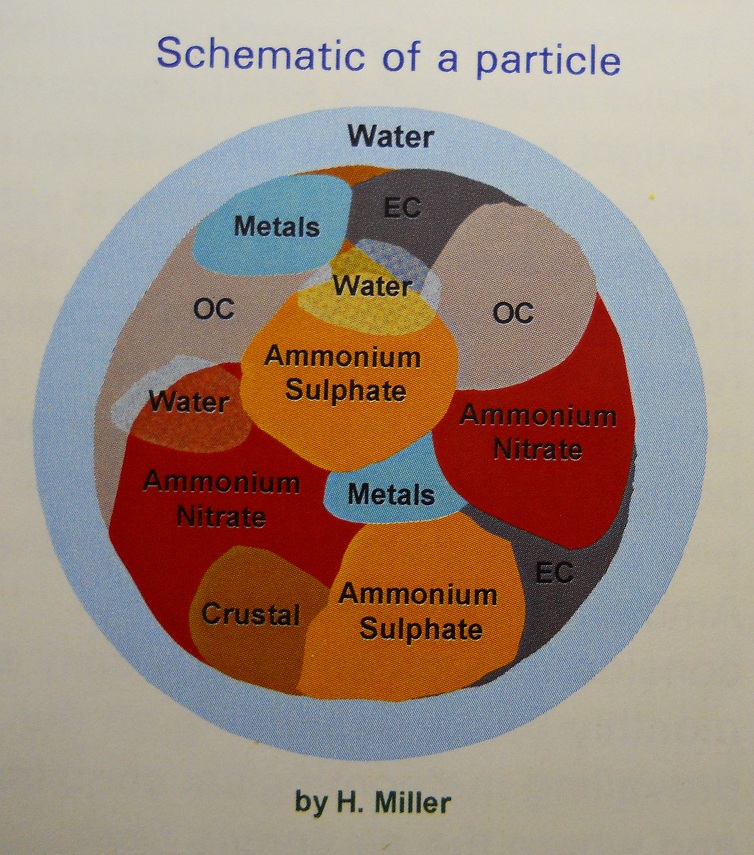
NATURE OF PARTICULATE MATTER
Particulate matter (PM) represents the collection of very
tiny liquid and solid particles that are suspended in the air. Individual particles are typically composed
of a very complex mixture of chemical species, and some particles are also
carriers of known toxic substances, such as polycyclic aromatic hydrocarbons,
some of which are known carcinogens.
Many particles have a solid core surrounded by a liquid layer.
Particles come in a variety of sizes. Two broadly monitored size fractions are
particles with diameter less than or equal to 10 micrometres (µm), known as inhalable
particles (or PM10), and those with diameter less than or
equal to 2.5 µm, known as fine particles (PM2.5).
PM is emitted directly to the air (primary PM), and it also
forms in the air (secondary PM) from precursor gases such SO2, NOx,
VOC and NH3. Sources of
primary PM include soot (elemental carbon, or EC) emitted
directly from combustion of fossil fuels; metals such as iron, lead, mercury and
cadmium; elements of soil and road dust; bio-aerosols (i.e. particles
containing or composed of living micro-organisms such as fungal spores and
mould); and salt (e.g. road salt and oceanic sea-salt).
Secondary PM includes:
ammonium sulphate (produced in the air from emissions of SO2
and NH3); ammonium nitrate (produced in the
air from emissions of NOx and NH3); and numerous
carbon-containing substances (known as organic carbon, or OC), which may be
emitted directly or formed in the air from emissions of VOC.
PM is a very complex pollutant, not only because particles
typically consist of a mixture of substances, but also because some of the
substances that make up the particles are semi-volatile. Semi-volatile substances can exist in the air
both as particles and vapours (i.e. gases).
The mass of semi-volatile PM (e.g. ammonium nitrate and some organic
compounds) is not static but can instead change frequently as the substances
respond to the changing meteorological, physical and chemical conditions that
they encounter while moving through the air.
Ambient levels of particles can be elevated year-round, and
in urban areas the levels are typically higher in the mornings and evenings
reflecting traffic patterns. Particles
can travel very large distances and affect areas thousands of kilometres away
from the sources of the emissions.