NATURE OF OZONE
Ozone (O3) is a different form of the familiar
oxygen (O2). Ozone levels
broadly increase with height to reach a maximum at approximately 25 km above
the Earth’s surface in what is known as the ozone layer, located in
the layer of the atmosphere known as the stratosphere. Because all ozone absorbs the harmful
ultraviolet rays emitted by the sun, stratospheric ozone is essential. At ground level, however, ozone is considered
a pollutant because it also causes adverse health and environmental effects.
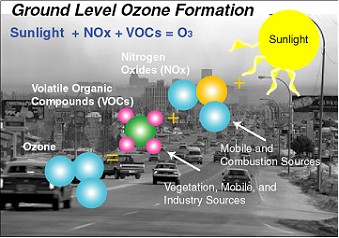
Ozone forms in the air during daylight hours. In the layer of the atmosphere which is in
contact with the Earth’s surface (i.e. the troposphere), it forms from complex
reactions involving precursor pollutants with the most important being NOx
and VOC.
Ambient ozone levels vary considerable on an hourly, daily
and monthly basis, depending on the prevailing meteorological conditions and
where the air comes from. Stratospheric
ozone can also at times be brought down to the surface and contribute to the
ambient ozone levels.
In many parts of Canada, the short-term (1-to 8-hour
averages) peak ozone levels produced from NOx and VOC are typically the highest
in the summer months because ozone formation is favoured by strong sunlight and
high air temperatures. Monthly average
levels, however, are typically the highest in spring months. Like PM 2.5, ozone can be
transported by the winds over large distances and affect areas hundreds to
thousands of kilometres away from the sources of the precursors.
Ground-level Ozone
Ground-level ozone forms in the air following the
dissociation of nitrogen dioxide (NO2). As NO2 absorbs sunlight, it splits
into nitric oxide (NO) and an unstable form of oxygen (O), which immediately
merges with the familiar oxygen (O2) to form ozone (O3).
NO2 and NO (known as nitrogen oxides, or NOx)
are emitted by the same sources. However, most of the ambient NO2 is
actually formed in the air from the conversion of the emitted NO.
The conversion of NO to NO2 occurs when NO reacts
with other substances, such as ozone. In
addition to the generation of NO2, the reaction of ozone and NO is
also a process (known as ozone scavenging) through which
ozone is removed from the air, since during the reaction ozone converts to oxygen
(O2).
NO, NO2 and ozone are interrelated. If the air contained only these three
substances, a cycle of ozone formation and scavenging would form, leading to
equilibrium between the three substances, and resulting in ozone levels which
would be relatively low.
The presence of VOC, however, disrupts this equilibrium
since VOC provide a pathway for NO to convert to NO2 without
scavenging ozone. With NO2 now
also being formed from reactions involving NO and VOC, the formed ozone can
accumulate in the air, thereby leading to significantly higher ozone levels
than would occur from the NOx-ozone equilibrium alone.
Ground-level Ozone
Effects of Reductions
in Ambient NO
Reductions in NOx emissions in urban areas that
cause a decrease in the local ambient NO levels can cause an increase in local
ozone levels because of the resulting decrease in the amount of ozone
scavenged. This effect may be more pronounced
in urban areas which are affected by ozone that is transported into the
area. Downwind from the urban area,
however, the reductions in emissions could lead to less ozone formation and
contribute to decreasing ozone levels.


